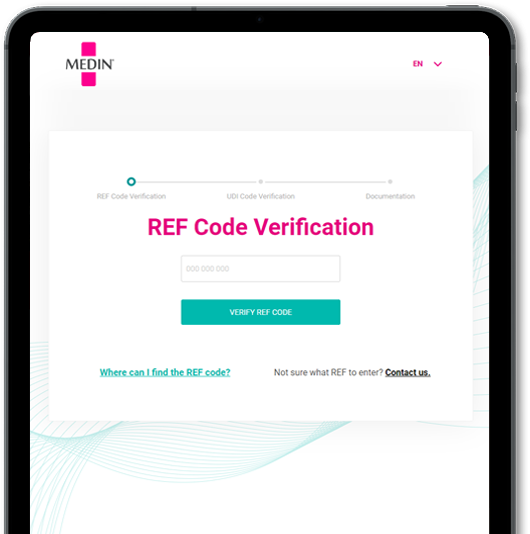
DocuLIV - electronic Information For use (eIFU)
Solution for digitizing paper manuals and other product information. Complies with EU requirements, reduces costs and environmental impact, and increases document accessibility through advanced cloud technology.
Main advantages of DocuLIV
eIFU - Electronic Instructions for Use
What is eIFU and Why is it Needed
eIFU (Electronic Instructions for Use) is a modern approach to providing product information and manuals in digital form instead of traditional paper.
This procedure is required by EU regulatory requirements for manufacturers of medical devices and in vitro diagnostic devices, aiming to reduce environmental impact and ensure high availability of product information.


What are the Benefits of eIFU
Implementing DocuLIV eIFU solution has numerous advantages beyond meeting EU regulatory requirements, not just for medical device manufacturers.
- Cost-effectiveness – eliminating the need for printed manuals saves manufacturers printing, packaging, and distribution costs.
- Up-to-date information – electronic manuals can be quickly updated to reflect the latest safety information, usage instructions, and regulatory changes.
- Availability and Trustworthiness – online availability combined with digital signatures and timestamps ensures easy access and verification of the origin and timing of each document.
Regulatory Framework
The implementation of eIFU is governed by several key EU regulations. Primarily, it is Regulation (EU) 2021/2226, which sets out detailed rules for providing electronic instructions for use of medical devices.
This regulation is in line with the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR), ensuring that eIFU maintain or improve safety and usability standards compared to paper instructions.
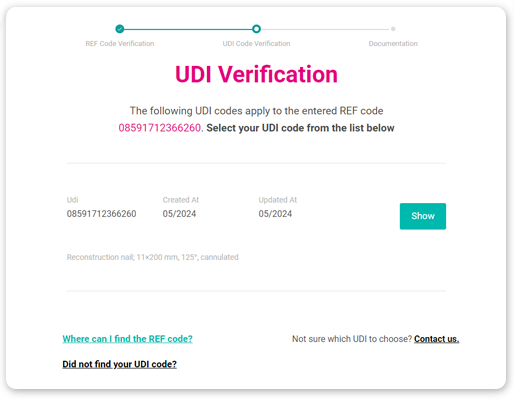
How to Implement DocuLIV eIFU Solution
DocuLIV is a complete cloud solution that can be implemented quickly without costs associated with purchasing special hardware. Our consultants will guide you through the entire process of digitizing product information and, if desired, will also provide integration with your information systems using the DocuLIV API, enabling fully automated data updates. Implementation typically occurs in 4 steps.
We Conduct an Audit of Existing Processes
We describe how you work with product information, analyze the current level of its digitization and security, and propose an eIFU implementation procedure.
We Deploy the DocuLIV Solution
DocuLIV runs in the cloud with high availability, 24/7 monitoring, and data security. Clients access the system on their domain, e.g., doculiv.yourdomain.com.
We Connect Data Sources
Product information needs to be linked with manufacturing information and ensure that product packaging is clearly marked with the corresponding QR code and URL.
We Launch and Manage DocuLIV
After performing integration and security tests, we launch the system into live operation and take care of its operation, backup, and regular updates.
How DocuLIV Works
Manufacturers upload product information into the DocuLIV system, which includes manuals, certificates, as well as training materials and manufacturing data identifying the type, batch, or serial number. The system then allows linking manufacturing data with product information and can generate materials for product packaging labels.
Customers or users can always download the latest product information with knowledge of the type and serial number. DocuLIV enables both updates and versioning of product information, so one type of product may have different manuals depending on the batch or serial number. All actions, updates, and operations with product information are logged in the system. The inserted product information can also be timestamped and digitally signed.
What You Gain by Implementing DocuLIV

Regulatory Compliance
Ensures compliance with the regulatory requirements of Regulation (EU) 2021/2226 and ISO 27001 safety standards.

Multilingual Support
Supports 24 language versions for seamless operation in all EU countries. Additional languages can be added.

Custom Design and Domain
User-friendly interface in corporate colors and design on your own domain II or III.
Cost Reduction
Manufacturers save on printing, packaging, and distribution costs of manuals and other product information.

High Availability
Product information is available anytime and anywhere with an internet connection.

Support and Partnership
We provide operation, technical support, regular updates, and data protection.
Our Satisfied Clients Speak for Us
Experience with DocuLIV in Medin, a.s.
“The implementation of the DocuLIV system solved the entire eIFU issue for us in terms of regulatory compliance and significantly improved the availability of product information for our customers and patients.”
“By digitizing product information, we have also reduced costs and gained the ability to quickly and efficiently update them. Therefore, I can recommend DocuLIV to anyone facing the challenge of implementing eIFU.”
Miroslav Havlíček
CEO MEDIN, a.s.
How Much Does the Implementation and Operation of DocuLIV Cost
The exact implementation cost can only be calculated with knowledge of all technical and process parameters for each client. To better orient you in the offer, we have created three basic DocuLIV operation packages corresponding to the needs of typical customer groups.
Basic
For Small Manufacturers
- Process Audit
- Integration of Data Sources
- Custom Tool Design in Corporate Colors
- Operation on Own Domain III
- Manual Data Updates
- Pre-launch Testing
- 20GB of Disk Space
- Backup to Two Geographically Separate Locations
- Firewall and Bot Protection
- Versioning and Logging of All Document Changes
- 24/7 Monitoring and 8/5 Support
- Regular Updates
from 5,980 CZK
excluding VAT / month
Pro
For Medium Manufacturers
- Process Audit
- Integration of Data Sources
- Custom Tool Design in Corporate Colors
- Operation on Own Domain III
- Pre-launch Testing
- 50GB of Disk Space
- Backup to Two Geographically Separate Locations
- Firewall and Bot Protection
- Versioning and Logging of All Document Changes
- Logging and Access Statistics for Documents
- 24/7 Monitoring and 8/5 Support
- Regular Updates
from 10,980 CZK
excluding VAT / month
Enterprise
For Large Manufacturers
- Process Audit
- Integration of Data Sources
- Custom Tool Design in Corporate Colors
- Operation on Own Domain II or III
- Automatic Data Update via API
- Documents Signed with a Digital Certificate
- Documents Timestamped
- Support for e-labeling
- Pre-launch Testing
- 100GB of Disk Space
- Backup to Two Geographically Separate Locations
- Firewall and Bot Protection
- Versioning and Logging of All Document Changes
- Logging and Access Statistics for Documents
- 24/7 Monitoring and 8/7 Support
- Regular Updates
from 18,980 CZK
excluding VAT / month
The basic implementation tool price ranges between 40,000 CZK and 300,000 CZK (one-time fee), depending on the complexity of data integration and special customer requests.
Interested in DocuLIV?
Great, we are glad to hear that, write to us or call us. During the first consultation, which is free, we will find out all the necessary information needed to prepare a calculation and plan for the implementation of eIFU in your company, and then we will agree on the next steps.
+420 533 433 927
Jan Janča
Founder